About Transcript One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan. Questions Tips & Thanks
Asma Afzal – Concept of Mole and Avogadro’s Number | Facebook
Jun 8, 20221 amu = 1/6.02214076 x 10 23 grams. This relationship means that if we had Avogadro’s number, or one mole, of carbon-12 atoms (which has an atomic weight of 12 amu by definition), that sample of carbon-12 would weigh exactly 12 grams. Chemists use this relationship to easily convert between the measurable unit of a gram and the invisible unit

Source Image: alamy.com
Download Image
This general chemistry video tutorial focuses on avogadro’s number and how it’s used to convert moles to atoms. This video also shows you how to calculate t

Source Image: americanscientist.org
Download Image
What is Mole in Simple Words | Avogadro’s Number and Mole | How did you convert moles to grams? – YouTube Avogadro’s number, or Avogadro’s constant, is the number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon-12.This experimentally determined value is approximately 6.0221 x 10 23 particles per mole. Avogadro’s number may be designated using the symbol L or N A.Note that Avogadro’s number, on its own, is a dimensionless quantity.

Source Image: youtube.com
Download Image
Avogadro’S Number Is The Number Of Grams In A Mole
Avogadro’s number, or Avogadro’s constant, is the number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon-12.This experimentally determined value is approximately 6.0221 x 10 23 particles per mole. Avogadro’s number may be designated using the symbol L or N A.Note that Avogadro’s number, on its own, is a dimensionless quantity. This number (6.02 x 1023) comes from the number of atoms in 12 g of carbon-12 (this is the carbon isotope with six protons and six neutrons). So, we can say that one mole of protons has a mass of one gram, and one mole of neutrons has a mass of one gram, as protons and neutrons have similar masses. • One mole of 1H atoms has a mass of one gram.
The mole and Avogadro’s number | Moles and molar mass | High school chemistry | Khan Academy – YouTube
Jul 7, 2023Convert from mass to moles by dividing the mass given by the compound’s molar mass. Convert from moles to molecules by multiplying the number of moles by Avogadro’s number. Solution: A The molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in Example 3.4.1: Avogadro’s Constant – EWT

Source Image: energywavetheory.com
Download Image
02 – What is Avogadro’s Number & the Mole in Chemistry? Part 1 – YouTube Jul 7, 2023Convert from mass to moles by dividing the mass given by the compound’s molar mass. Convert from moles to molecules by multiplying the number of moles by Avogadro’s number. Solution: A The molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in Example 3.4.1:

Source Image: m.youtube.com
Download Image
Asma Afzal – Concept of Mole and Avogadro’s Number | Facebook About Transcript One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan. Questions Tips & Thanks

Source Image: facebook.com
Download Image
What is Mole in Simple Words | Avogadro’s Number and Mole | How did you convert moles to grams? – YouTube This general chemistry video tutorial focuses on avogadro’s number and how it’s used to convert moles to atoms. This video also shows you how to calculate t

Source Image: youtube.com
Download Image
What is the Relationship between a Mole and Avogadro’s number | Molecules, Mole, Chemistry notes 11 days ago Scientific notation is used to represent huge numbers in a concise and easy way. With huge magnitudes involved in many science problems (like the mole), it would become very tiresome to write out so many numbers. For instance 1 mole in in standard form would look like this:

Source Image: pinterest.com
Download Image
The Mole Concept Avogadro’s Number = x ppt download Avogadro’s number, or Avogadro’s constant, is the number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon-12.This experimentally determined value is approximately 6.0221 x 10 23 particles per mole. Avogadro’s number may be designated using the symbol L or N A.Note that Avogadro’s number, on its own, is a dimensionless quantity.

Source Image: slideplayer.com
Download Image
Chemistry Lesson: The Mole (Avogadro’s Number) – Get Chemistry Help This number (6.02 x 1023) comes from the number of atoms in 12 g of carbon-12 (this is the carbon isotope with six protons and six neutrons). So, we can say that one mole of protons has a mass of one gram, and one mole of neutrons has a mass of one gram, as protons and neutrons have similar masses. • One mole of 1H atoms has a mass of one gram.
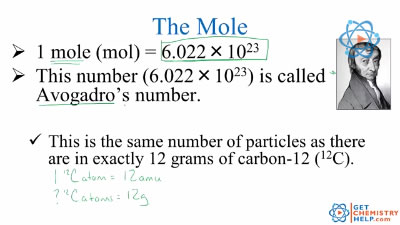
Source Image: getchemistryhelp.com
Download Image
02 – What is Avogadro’s Number & the Mole in Chemistry? Part 1 – YouTube
Chemistry Lesson: The Mole (Avogadro’s Number) – Get Chemistry Help Jun 8, 20221 amu = 1/6.02214076 x 10 23 grams. This relationship means that if we had Avogadro’s number, or one mole, of carbon-12 atoms (which has an atomic weight of 12 amu by definition), that sample of carbon-12 would weigh exactly 12 grams. Chemists use this relationship to easily convert between the measurable unit of a gram and the invisible unit
What is Mole in Simple Words | Avogadro’s Number and Mole | How did you convert moles to grams? – YouTube The Mole Concept Avogadro’s Number = x ppt download 11 days ago Scientific notation is used to represent huge numbers in a concise and easy way. With huge magnitudes involved in many science problems (like the mole), it would become very tiresome to write out so many numbers. For instance 1 mole in in standard form would look like this: