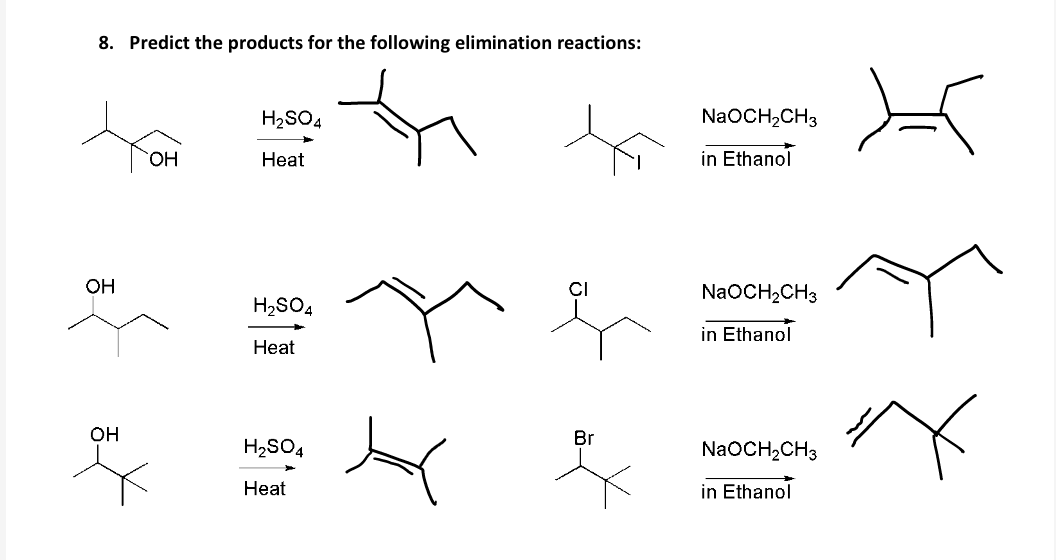
Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. It states that in an elimination reaction the major product is the more stable alkene with the more highly substituted double bond. This situation is illustrated by the 2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.
Week 7 Predict Outcomes – Kate Graham.pdf – Week 7 -Predicting Reaction Type Practice Determining Mechanism and Product • • Use the flowchart page 3 | Course Hero
I realize that since the reagent is a strong nucleophile & a strong base, it can only participate in either an $\ceE2$ reaction or in a combination of the $\ceE2$ and $\ceS_N2$ reactions, depending on the substrate. I think the substrate is secondary, so $\ceE2$ should predominate and $\ceS_N2$ should give a minor product.
Source Image: chegg.com
Download Image
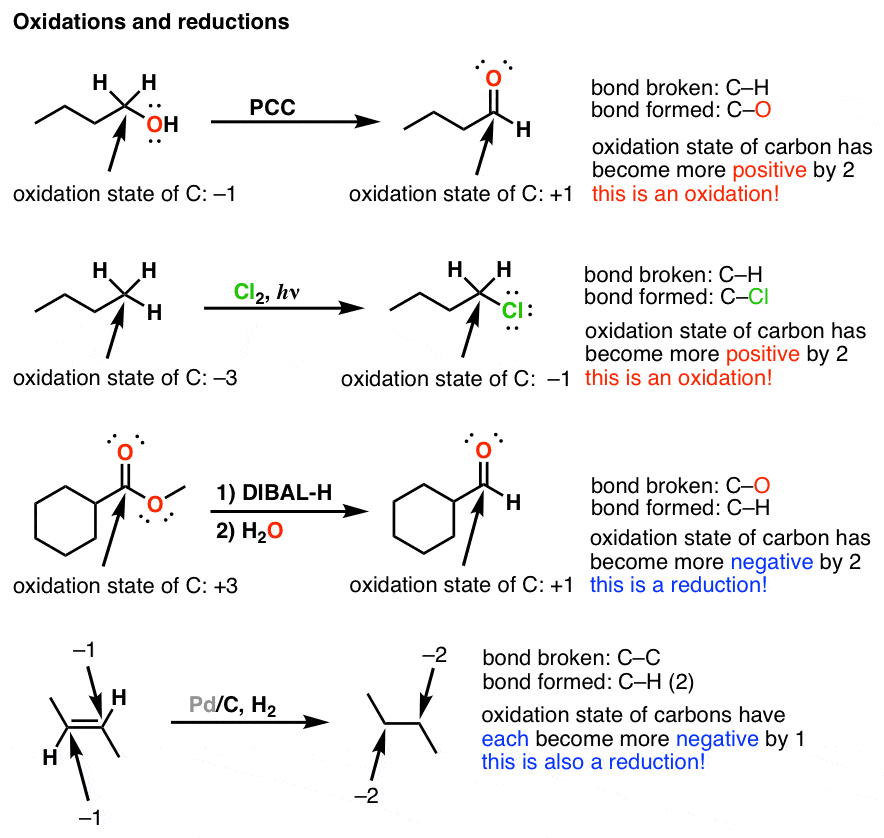
Trends in alkene stability. Zaitsev’s rule and a generalization thereof. Applications of Zaitsev’s general rule to site selectivity and stereoselectivity in
Source Image: masterorganicchemistry.com
Download Image
7.9 Predicting the Products of Elimination Reactions – YouTube
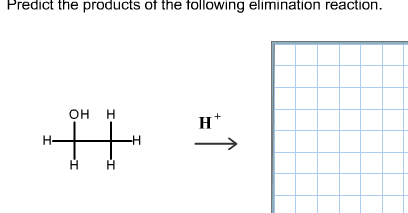
Chemistry Chemistry questions and answers Predict the products of the following elimination reaction. This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Predict the products of the following elimination reaction.
Source Image: chegg.com
Download Image
Predict The Products Of The Elimination Reaction
Chemistry Chemistry questions and answers Predict the products of the following elimination reaction. This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Predict the products of the following elimination reaction.
Unit 13 Amines Unit 14 Spectroscopy Science Organic chemistry Unit 5: Substitution and elimination reactions About this unit Sn1, Sn2, E1, and E2 reactions form the basis for understanding why certain products are more likely to form than others.
Solved Predict the products of the following elimination | Chegg.com
Elimination reactions usually occur such that they are removing a hydrogen from the carbon attached to the fewest hydrogens. This is called “Zaitsev’s rule”. So when you form an alkene in an elimination reaction, make sure you form the most substituted alkene (i.e. the one with the most carbon atoms directly attached).
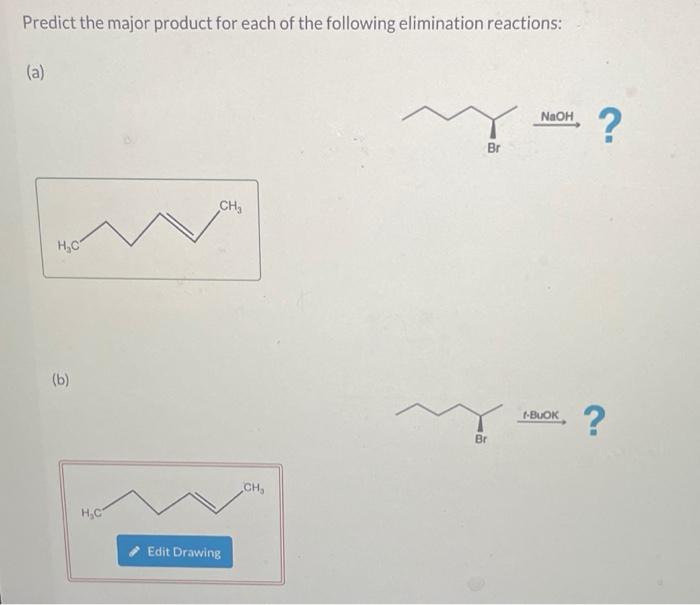
Solved Predict the major product for each of the following | Chegg.com
Source Image: chegg.com
Download Image
Spontaneous elimination of halides from β-halo carbanions upon geometry optimizations: Computational predictions of the outcomes from elimination reactions proceeding via an E1cb-Like mechanism – ScienceDirect
Elimination reactions usually occur such that they are removing a hydrogen from the carbon attached to the fewest hydrogens. This is called “Zaitsev’s rule”. So when you form an alkene in an elimination reaction, make sure you form the most substituted alkene (i.e. the one with the most carbon atoms directly attached).

Source Image: sciencedirect.com
Download Image
Week 7 Predict Outcomes – Kate Graham.pdf – Week 7 -Predicting Reaction Type Practice Determining Mechanism and Product • • Use the flowchart page 3 | Course Hero
Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. It states that in an elimination reaction the major product is the more stable alkene with the more highly substituted double bond. This situation is illustrated by the 2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.

Source Image: coursehero.com
Download Image
7.9 Predicting the Products of Elimination Reactions – YouTube
Trends in alkene stability. Zaitsev’s rule and a generalization thereof. Applications of Zaitsev’s general rule to site selectivity and stereoselectivity in

Source Image: m.youtube.com
Download Image
Predict the product(s) for each elimination reaction below. | Homework.Study.com
Elimination reactions Alkyl halides undergo elimination via two common mechanisms, known as E2 and E1, which show some similarities to S 2 and S 1, respectively. In E2, elimination shows a second order rate law, and occurs in a single concerted step (proton abstraction at C α occurring at the same time as Cβ-X bond cleavage).

Source Image: homework.study.com
Download Image
A Level H2 Chemistry Tuition and notes Singapore
Chemistry Chemistry questions and answers Predict the products of the following elimination reaction. This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Predict the products of the following elimination reaction.

Source Image: alevelchemistrysg.com
Download Image
Accurately Predicting Protein pKa Values Using Nonequilibrium Alchemy | Journal of Chemical Theory and Computation
Unit 13 Amines Unit 14 Spectroscopy Science Organic chemistry Unit 5: Substitution and elimination reactions About this unit Sn1, Sn2, E1, and E2 reactions form the basis for understanding why certain products are more likely to form than others.

Source Image: pubs.acs.org
Download Image
Spontaneous elimination of halides from β-halo carbanions upon geometry optimizations: Computational predictions of the outcomes from elimination reactions proceeding via an E1cb-Like mechanism – ScienceDirect
Accurately Predicting Protein pKa Values Using Nonequilibrium Alchemy | Journal of Chemical Theory and Computation
I realize that since the reagent is a strong nucleophile & a strong base, it can only participate in either an $\ceE2$ reaction or in a combination of the $\ceE2$ and $\ceS_N2$ reactions, depending on the substrate. I think the substrate is secondary, so $\ceE2$ should predominate and $\ceS_N2$ should give a minor product.
7.9 Predicting the Products of Elimination Reactions – YouTube A Level H2 Chemistry Tuition and notes Singapore
Elimination reactions Alkyl halides undergo elimination via two common mechanisms, known as E2 and E1, which show some similarities to S 2 and S 1, respectively. In E2, elimination shows a second order rate law, and occurs in a single concerted step (proton abstraction at C α occurring at the same time as Cβ-X bond cleavage).